San Diego, Calif., June 28, 2021 – Shoreline Biosciences, Inc. (Shoreline), a biotechnology company developing intelligently designed allogeneic off-the-shelf, standardized, and targeted induced pluripotent stem cells (iPSC) derived natural killer (NK) and Macrophage cellular immunotherapies, today announced the appointment of Mohammad El-Kalay, Ph.D., as Senior VP and Head of CMC (Chemistry, Manufacturing, and Control).
Dr. El-Kalay brings over three decades of experience in global biopharmaceutical operations to Shoreline, including key leadership roles in cell therapy companies where he led the successful translation of several cell therapy candidates into late-stage clinical development, as well as developing a T-cell purging device that was later marketed. Dr. El-Kalay will be responsible for overseeing Shoreline’s manufacturing, technical, and supply chain operations.
“We are thrilled to welcome Mohammad as SVP and Head of CMC at such a pivotal time in the growth of Shoreline,” said William Sandborn MD, Shoreline Chief Medical Officer. “Mohammad’s extensive technical expertise in process development and manufacturing of cell therapies, combined with his executive management experience, will benefit us greatly as we advance our emerging pipeline of cell therapy candidates toward the clinic.”
Dr. El-Kalay joins Shoreline from Poseida Therapeutics, where he was VP, Technical Operations and was responsible for developing processes to manufacture their autologous and allogeneic CAR-T cell products. Prior to Poseida, he was SVP, Technical Operations for San Bio, Inc., directing development of manufacturing processes of their gene modified allogeneic mesenchymal stem cells to treat CNS diseases. Before that, he held senior technical and leadership roles at Sangamo Therapeutics, Stem Cells, Inc., and was Founder, President, and CEO of EyeCyte, an autologous cell therapy company. He also held senior leadership R&D and Technical Operation roles at MicroIslet, Telos Pharmaceuticals, MorphoGen Pharmaceuticals, Osiris Therapeutics, SyStemix, and Applied ImmuneSciences. Dr. El-Kalay holds a B.Sc., M.Sc. and Ph.D. from Strathclyde University in Glasgow, UK.
“This is an exciting time to join the Shoreline team as it advances its core iPSC cell therapy technologies with the support of well-known biotech investors and two major partnerships with industry leaders Kite and BeiGene,” commented Dr. El-Kalay. “I look forward to leveraging my thirty years of experience to develop and commercialize next-generation iPSC-derived allogeneic cell immunotherapies for cancer.”
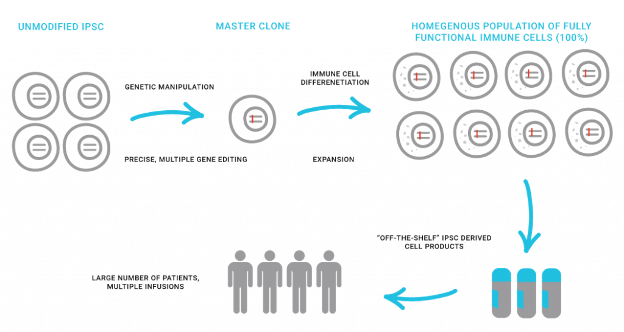
About Shoreline’s iPSC NK cell and Macrophage Technology Platform
Shoreline has developed a proprietary platform focused on iPSC-derived natural killer (NK) cells and macrophages that are optimized with precise and rational genetic reprogramming. The Shoreline NK cell and macrophage-based cell therapies are designed to provide an effective and efficient means for targeting and killing tumors as well as repairing tissue homeostasis. Shoreline’s approach, based on the advantage of its iPSC cell engineering and expansion, is being used to create a streamlined, affordable, and scalable manufacturing process that can deliver cell therapy treatments to patients in a more cost-effective, time-saving manner.
Shoreline Biosciences Investor and Media Contact:
Amy Conrad
Juniper Point
858-366-3243