— Partnership Follows Kite’s Investment in Shoreline’s Recent Series A Financing —
Santa Monica, Calif., and San Diego, June 17, 2021 – Kite, a Gilead Company (Nasdaq: GILD), and Shoreline Biosciences, Inc. (Shoreline), a biotechnology company developing intelligently designed allogeneic off-the-shelf, standardized and targeted natural killer (NK) and Macrophage cellular immunotherapies derived from induced pluripotent stem cells (iPSC) for cancer and other serious diseases, today announced a strategic partnership to develop novel cell therapies across a variety of cancer targets.
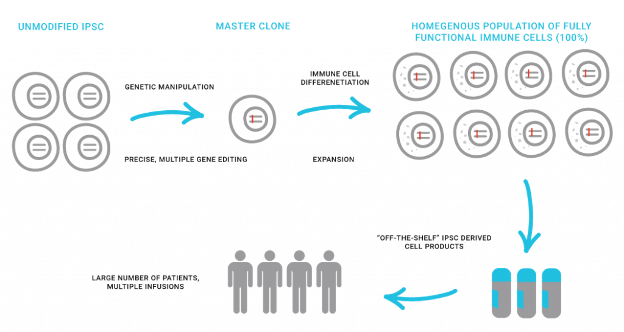
The collaboration will leverage Shoreline’s deep expertise in iPSC differentiation and genetic reprogramming in combination with Kite’s extensive cell therapy development, commercialization and manufacturing expertise to develop novel allogeneic candidates for a range of hematologic malignancies. The collaboration will focus initially on chimeric antigen receptor (CAR) NK targets, with Kite having an option to expand the collaboration to include an iPSC CAR Macrophage program for an undisclosed target to be selected post deal execution. This agreement follows Kite’s investment in Shoreline’s recent Series A financing.
“As the leader in cell therapy, we are focused on investing in and delivering on the most promising opportunities to further optimize the therapeutic potential of cell therapy,” said Mert Aktar, Vice President of Corporate Development and Strategy at Kite. “We are excited about the potential of Shoreline’s next-generation approach to allogeneic development, and how our collaboration can accelerate this research across different leukemias and lymphomas.”
“We’re thrilled to strengthen our relationship with Kite, which has been at the forefront of cancer immunotherapy for over a decade, through this strategic partnership,” said Kleanthis G. Xanthopoulos, Ph.D., Shoreline Co-Founder, Chairman and CEO. “The combined strength of Kite’s leadership in CAR T-cell therapies and our cutting-edge iPSC platform will potentially accelerate Shoreline’s timeline to the clinic, expand our pipeline opportunities and deliver transformational treatment options for cancer.”
Under the terms of the agreement, Shoreline will receive an upfront payment and will be eligible to receive additional payments totaling over $2.3 billion as well as royalties based on achievement of certain development and commercial milestones.
About Shoreline Biosciences
Shoreline is a biotechnology company dedicated to advancing the development of intelligently designed allogeneic off-the-shelf, targeted and standardized cellular immunotherapies for cancer and other serious diseases. Shoreline is building a pipeline of natural killer (NK) cell and macrophage-cell therapy candidates derived from its deep expertise in iPSC differentiation methods and genetic reprogramming of disease relevant pathways. Shoreline has a strategic manufacturing relationship with the Advanced Cell Therapy Laboratory and is supported by high-quality institutional investors including Boxer Capital, BVF Partners L.P., Commodore Capital, Cormorant Capital, Janus Henderson Investors, Logos Capital, Kite, a Gilead Company, Wedbush Healthcare Partners, Stork Capital and others. Shoreline Biosciences is headquartered in San Diego, CA.
For more information, please visit https://shorelinebio.com/ and engage with us on LinkedIn.
About Kite
Kite, a Gilead Company, is a global biopharmaceutical company based in Santa Monica, California, with commercial manufacturing operations in North America and Europe. Kite’s singular focus is cell therapy to treat and potentially cure cancer. As the cell therapy leader, Kite has more approved CAR T indications to help more patients than any other company. For more information on Kite, please visit www.kitepharma.com.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis and cancer. Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California.
Gilead and Kite Forward-Looking Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the risk that Kite may not realize the anticipated benefits of this collaboration with Shoreline; difficulties or unanticipated expenses in connection with the collaboration and the potential effect on Kite’s earnings; and the possibility that the parties may make a strategic decision to terminate this collaboration at any time. These and other risks, uncertainties and other factors are described in detail in Gilead’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2021, as filed with the U.S. Securities and Exchange Commission. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties and are cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements are based on information currently available to Kite and Gilead, and Kite and Gilead assume no obligation and disclaim any intent to update any such forward-looking statements.
Kite, the Kite logo and GILEAD are trademarks of Gilead Sciences, Inc. or its related companies.
For more information on Kite, please visit the company’s website at www.kitepharma.com or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000. Follow Kite on social media on Twitter (@KitePharma) and LinkedIn.
Gilead and Kite Contacts:
Jacquie Ross, Investors
(650) 358-1054
Nathan Kaiser, Media
(650) 522-1853
Shoreline Contact:
Amy Conrad
amy@juniper-point.com
858-366-3243